On Tuesday, December 22, 2020, Brian Evans (CEO) and Dr. Bob Weissinger (CMO), received the first vaccination of the Moderna COVID-19 vaccine at Clarke County Hospital. 29 additional CCH healthcare workers received their first dose of the vaccine later that afternoon. The vaccine requires a second dose 28 days later to achieve optimal effectiveness in protecting against the Coronavirus. We received our initial delivery of 100 doses and we expect to administer 80+ vaccines to our team by Christmas day.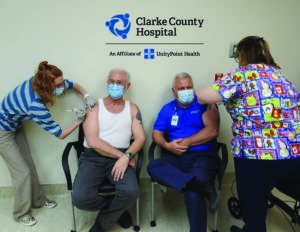
The Moderna vaccine is being distributed in the US one week after the delivery of the Pfizer vaccine.
“To have two vaccines against a novel virus authorized and distributed within a year is extraordinary and should be a great source of pride for every member of the HHS family and every American. Authorization of Moderna’s vaccine means we can accelerate the vaccination of frontline healthcare workers and Americans in long-term-care facilities, and, ultimately, bring a faster end to this pandemic.” – Health and Human Services (HHS) Secretary Alex Azar.
The Moderna vaccine underwent strict Food and Drug Administration (FDA) approval protocol, but the process was accelerated due to the dire need for a coronavirus vaccine. The FDA gave Emergency Use Authorization for the Moderna Vaccine on Friday, December 18.
Distribution, through Operation Warp Speed, began immediately after the FDA Approval.
Like the Pfizer vaccine, the Moderna product is an mRNA vaccine that stimulates the immune system. Some vaccine facts:
- Like all vaccines, COVID-19 mRNA vaccines have been rigorously tested for safety before being authorized for use in the United States.
- mRNA technology is new but not unknown. They have been studied for more than a decade.
- mRNA vaccines do not contain a live virus and do not carry a risk of causing disease in the vaccinated person.
- mRNA from the vaccine never enters the nucleus of the cell and does not affect or interact with a person’s DNA.
Side effects from taking the vaccine result from our immune system working to develop immunity to COVID-19 and can include injection site stiffness or soreness, body aches, fatigue, and/or a minor fever. If experienced, side effects are considered minor and can last up to 24 – 48 hours.
Statewide administration of the COVID-19 vaccines is being approached using a “phased” group process:
Phase 1a: Health care personnel, long term care facility staff, and residents
Phase 1b: Essential workers (ex: education sector, food and agriculture, utility workers, law enforcement, firefighters, transportation industry)
Phase 1c: Adults with high-risk medical conditions and Adults 65 years of age and older
Additional group vaccination schedule information is expected from the CDC and Iowa Department of Public Health, guided by the progress of the Phase 1 vaccinations and the availability of the vaccines, to be announced in the near future.
Administration of the vaccines is the first step in gaining control over the COVID-19 virus. The CDC estimates that at least 70% of the population needs to receive the vaccine before we can begin to control the spread of COVID-19.
Consult with your physician prior to receiving a vaccination for additional guidance if:
- You are pregnant or considering pregnancy in the near future
- You have had a positive COVID-19 test within the last 90 days.
- Have severe allergies
- Have previously had an adverse reaction to vaccines
- You are under 18 years of age
Clarke County Hospital is encouraging community residents to participate in the vaccination program as soon as the vaccine becomes available for widespread administration. The science behind the vaccine is strong, and the resulting positives related to taking the vaccine are undeniable.
